how to analyze uv aborptions|how to use uv absorption : manufacturer you might have a quantum mechanical picture of light absorption, which emphasizes that light energy comes in quantized units, called photons, and that a molecule’s energy also comes in quantized units or “quanta”, so when a molecule absorbs a photon, it takes up the photon’s energy to reach an “excited state” of some sort.
WEBPersona 4 – PS2 PTBR. Persona 4 PTBR situa-se dois anos após os eventos de Persona 3 (2009), na cidade interiorana fictícia de Inaba, onde um grupo de adolescentes passa a investigar uma série de assassinatos relacionados a um estranho nevoeiro. Cronologicamente, é o quinto episódio da série Persona, spin-off de Shin Megami Tensei.
{plog:ftitle_list}
Resultado da INTERPROPERTY SYSTEM (IPS) have been in the software industry for over 30 years. We are the creators of Prodev and PDS family of softwares. Both are well known and widely used in the business world, mainly as an appraisal software within the property industry. CLICK BELOW TO FIND OUT MORE.
Ultraviolet-visible (UV-vis) spectroscopy is used to obtain the absorbance spectra of a compound in solution or as a solid.This page takes a brief look at how UV-visible absorption spectra can be used .
computerised universal testing machine price in india
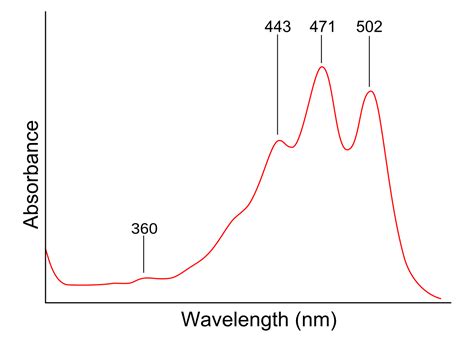
This page takes a brief look at how UV-visible absorption spectra can be used to help identify compounds and to measure the concentrations of coloured solutions. It assumes that you know how these spectra arise, and know what is meant .The diagram below shows a simple UV-visible absorption spectrum for buta-1,3-diene - a molecule we will talk more about later. Absorbance (on the vertical axis) is just a measure of the amount of light absorbed. The higher the value, the . Understanding UV-Vis Spectroscopy Will Make You More Fun At Parties. In today’s post we’ll discuss why most molecules are colourless, introduce the useful technique of UV-visible spectroscopy, and finally explain .
you might have a quantum mechanical picture of light absorption, which emphasizes that light energy comes in quantized units, called photons, and that a molecule’s energy also comes in quantized units or “quanta”, so when a molecule absorbs a photon, it takes up the photon’s energy to reach an “excited state” of some sort.
computerized universal tensile testing machine
Stepson how to analyze the UV-VIS spectrum are presented. Thispaper is expected to provide useful information forresearchers and novice students who are studying UV-VISspectrophotometry. Estimated .The C=O absorption of a ketone is almost always in the range 1680 to 1750 cm –1; the O–H absorption of an alcohol is almost always in the range 3400 to 3650 cm –1; the C=C absorption of an alkene is almost always in the range 1640 to 1680 cm –1; and so forth. By learning where characteristic functional-group absorptions occur, it’s . How To Interpret IR Spectra In 1 Minute Or Less: The 2 Most Important Things To Look For [Tongue and Sword] Last post, we briefly introduced the concept of bond vibrations, and we saw that we can think of covalent bonds as a bit like balls and springs: the springs vibrate, and each one “sings” at a characteristic frequency, which depends on the strength of the bond and . An absorption band is observed below the main absorption edge (at 300°K. the max. of this band is at 0.86 ev.); the absorption in this band increases with increasing temp. This band is due to excitons bound to neutral acceptors, and these are presumably the same ones that play a decisive role in the transport properties, which are considered .
computerized universal testing machine manufacturer in india
coefficient unique to the analyte measured. Often, UV-Visible absorption methods are used to analyze solution-phase samples, however, solid-state samples can also be readily measured using a variety of UV-Visible techniques. The same principles which govern solution-phase samples also apply to solid-state substances, however there are a few
Use the Analyze spectrum tool for analyzing the calculated spectra, graphical editing it and adding experimental ones. Usage. See above the following: Displaying spectra Two forms of Analyze Spectrum window Working with experimental spectra Spectra analysis Line shapes Graphic design of spectra diagrams Calculated Spectrum options & Energy shiftThe whole idea of UV spectroscopy is that different compounds might absorb photons of different wavelengths based on their electronic structures. We might be able to look at the UV spectrum of a compound and tell its identity or structure; that task would be especially straightforward if we had a few different options to choose from.The molar absorption coefficient is a measurement of how strongly a substance absorbs light. The larger its value, the greater the absorption. With larger conjugated systems, the absorption peak wavelengths tend to be shifted toward the long wavelength region and the absorption peaks tend to be larger.Based on the analysis of the absorption characteristics of the PMC, further fine tuning of the desired optical properties can be achieved by careful selection of the type and composition of nanofiller in a polymer matrix. . The UV–vis absorption spectra of the daunorubicin residue after cancer cells incubation reveal that MPA-CdTe QDs can .
UV-Vis spectroscopy is used to quantify the amount of DNA or protein in a sample, for water analysis, and as a detector for many types of chromatography. Kinetics of chemical reactions are also measured with UV-Vis spectroscopy by taking repeated UV-Vis measurements over time. UV-Vis measurements are generally taken with a spectrophotometer.
Great discussion of the topic!! Question on Azo yellow dye. When I look up the absorption spectrum of cis azo yellow on the internet, I see that the absorption peak at ~420 is much weaker than the peak further into the UV, which would be expected for an n->pi star transition for the reasons you explained, but the lambda max for the stronger shorter .

Quartz cuvettes are designed for use in UV-visible spectrophotometry. When handling the cuvette, avoid touching the sides the light will pass through (generally, . Choose and set the wavelength of light to .UV and visible absorption of transition metal complexes. Ultraviolet and visible absorption spectroscopy involve transitions between electron energy levels in atoms and molecules where the energy difference corresponds to the .
uv absorption spectroscopy formula
uv absorption spectroscopy
Understand the Beer-Lambert law for absorbance, A = ɛ x l x c. The standard equation for absorbance is A = ɛ x l x c, where A is the amount of light absorbed by the sample for a given wavelength, ɛ is the molar .When analyzing nanoparticles with absorption spectroscopy, the first absoption maximum represents the transition of electrons in the valence band to the conduction band, leaving a hole and thus .
Instrument Designs for Molecular UV/Vis Absorption. Filter Photometer. The simplest instrument for molecular UV/Vis absorption is a filter photometer (Figure 10.3.1 ), which uses an absorption or interference filter to isolate a band of radiation. The filter is placed between the source and the sample to prevent the sample from decomposing when exposed to higher .
uv absorption solutions
Ultraviolet-visible spectroscopy or ultraviolet-visible spectrophotometry (UV-Vis or UV/Vis) refers to absorption spectroscopy or reflectance spectroscopy in the ultraviolet-visible spectral region. Ultraviolet-visible (UV-VIS) spectroscopy is an analytical method that can measure the analyte quantity depending on the amount of light received .Infrared spectroscopy is used to analyze a wide variety of samples, but it cannot solve every chemical analysis problem. When used in conjunction with other methods such as mass spectroscopy, nuclear magnetic resonance, and elemental analysis, infrared spectroscopy usually makes possible the positive identification of a sample.If you're seeing this message, it means we're having trouble loading external resources on our website. If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.
Plots of the predicted UV/Visible spectrum for a molecule use this numeric data from each of the computed excited states. Conventionally, UV-Visible spectra area plotted as ε vs. λ (excitation wavelength in nm), and the peaks assume a Gaussian band shape. The equation of a Gaussian band shape is: [Equation 1]
tion and purity measurements is UV absorbance measurement with a spectrophotometer. OPTIMAL WORKING RANGE FOR CONCENTRATION AND RATIO DETERMINATION Suppliers of MVS instruments define the detection limits between 0.5 to 2 ng/µl, but warn that below 20 ng/µl the reliability of the purity ratios is compromised. It is often discussed UV–vis absorption spectroscopy is one of the most accessible spectroscopic techniques at the high school educational level, and it is usually introduced in analytical chemistry courses due to its high versatility and to the wide range of applications in many fields of chemistry. Within this framework, we have developed an easy-to-use “simulation tool” to identify and .
The best choice of wavelength for Beer's law analysis is one which is selective for the chosen analyte, has a large \({\epsilon}\) and where the absorption curve is relatively flat. As shown in Figure 9.4.1, a peak with a large \({\epsilon}\) will yield a better sensitivity than a peak with a smaller (\{\epsilon}\).
computerized universal testing machine pdf
computerized universal testing machine price
web24 de abr. de 2023 · O Ozempic é um medicamento análogo do GLP-1, hormônio naturalmente presente no intestino humano responsável por diminuir os níveis de glicose no sangue. O hormônio atua também no controle do peso, uma vez que tem como alvo locais do cérebro responsáveis pela saciedade. Liberado após refeições, o GLP-1 traz .
how to analyze uv aborptions|how to use uv absorption